SCI Publications
2018
G.A. Ateshian, J.J. Shim, S.A. Maas, J.A. Weiss.
“Finite Element Framework for Computational Fluid Dynamics in FEBio,” In Journal of Biomechanical Engineering, Vol. 140, No. 2, ASME International, pp. 021001. Jan, 2018.
DOI: 10.1115/1.4038716
The mechanics of biological fluids is an important topic in biomechanics, often requiring the use of computational tools to analyze problems with realistic geometries and material properties. This study describes the formulation and implementation of a finite element framework for computational fluid dynamics (CFD) in FEBio, a free software designed to meet the computational needs of the biomechanics and biophysics communities. This formulation models nearly incompressible flow with a compressible isothermal formulation that uses a physically realistic value for the fluid bulk modulus. It employs fluid velocity and dilatation as essential variables: The virtual work integral enforces the balance of linear momentum and the kinematic constraint between fluid velocity and dilatation, while fluid density varies with dilatation as prescribed by the axiom of mass balance. Using this approach, equal-order interpolations may be used for both essential variables over each element, contrary to traditional mixed formulations that must explicitly satisfy the inf-sup condition. The formulation accommodates Newtonian and non-Newtonian viscous responses as well as inviscid fluids. The efficiency of numerical solutions is enhanced using Broyden's quasi-Newton method. The results of finite element simulations were verified using well-documented benchmark problems as well as comparisons with other free and commercial codes. These analyses demonstrated that the novel formulation introduced in FEBio could successfully reproduce the results of other codes. The analogy between this CFD formulation and standard finite element formulations for solid mechanics makes it suitable for future extension to fluid–structure interactions (FSIs).
A. Erdemir, P.J. Hunter, G.A. Holzapfel, L.M. Loew, J. Middleton, C.R. Jacobs, P. Nithiarasu, R. Löhner, G. Wei, B.A. Winkelstein, V.H. Barocas, F. Guilak, J.P. Ku, J.L. Hicks, S.L. Delp, M.S. Sacks, J.A. Weiss, G.A. Ateshian, S.A. Maas, A.D. McCulloch, G.C.Y. Peng.
“Perspectives on Sharing Models and Related Resources in Computational Biomechanics Research,” In Journal of Biomechanical Engineering, Vol. 140, No. 2, ASME International, pp. 024701. Jan, 2018.
DOI: 10.1115/1.4038768
The role of computational modeling for biomechanics research and related clinical care will be increasingly prominent. The biomechanics community has been developing computational models routinely for exploration of the mechanics and mechanobiology of diverse biological structures. As a result, a large array of models, data, and discipline-specific simulation software has emerged to support endeavors in computational biomechanics. Sharing computational models and related data and simulation software has first become a utilitarian interest, and now, it is a necessity. Exchange of models, in support of knowledge exchange provided by scholarly publishing, has important implications. Specifically, model sharing can facilitate assessment of reproducibility in computational biomechanics and can provide an opportunity for repurposing and reuse, and a venue for medical training. The community's desire to investigate biological and biomechanical phenomena crossing multiple systems, scales, and physical domains, also motivates sharing of modeling resources as blending of models developed by domain experts will be a required step for comprehensive simulation studies as well as the enhancement of their rigor and reproducibility. The goal of this paper is to understand current perspectives in the biomechanics community for the sharing of computational models and related resources. Opinions on opportunities, challenges, and pathways to model sharing, particularly as part of the scholarly publishing workflow, were sought. A group of journal editors and a handful of investigators active in computational biomechanics were approached to collect short opinion pieces as a part of a larger effort of the IEEE EMBS Computational Biology and the Physiome Technical Committee to address model reproducibility through publications. A synthesis of these opinion pieces indicates that the community recognizes the necessity and usefulness of model sharing. There is a strong will to facilitate model sharing, and there are corresponding initiatives by the scientific journals. Outside the publishing enterprise, infrastructure to facilitate model sharing in biomechanics exists, and simulation software developers are interested in accommodating the community's needs for sharing of modeling resources. Encouragement for the use of standardized markups, concerns related to quality assurance, acknowledgement of increased burden, and importance of stewardship of resources are noted. In the short-term, it is advisable that the community builds upon recent strategies and experiments with new pathways for continued demonstration of model sharing, its promotion, and its utility. Nonetheless, the need for a long-term strategy to unify approaches in sharing computational models and related resources is acknowledged. Development of a sustainable platform supported by a culture of open model sharing will likely evolve through continued and inclusive discussions bringing all stakeholders at the table, e.g., by possibly establishing a consortium.
J.N. Todd, T.G. Maak, G.A. Ateshian, S.A. Maas, J.A. Weiss.
“Hip chondrolabral mechanics during activities of daily living: Role of the labrum and interstitial fluid pressurization,” In Journal of Biomechanics, Vol. 69, Elsevier BV, pp. 113--120. March, 2018.
DOI: 10.1016/j.jbiomech.2018.01.001
Osteoarthritis of the hip can result from mechanical factors, which can be studied using finite element (FE) analysis. FE studies of the hip often assume there is no significant loss of fluid pressurization in the articular cartilage during simulated activities and approximate the material as incompressible and elastic. This study examined the conditions under which interstitial fluid load support remains sustained during physiological motions, as well as the role of the labrum in maintaining fluid load support and the effect of its presence on the solid phase of the surrounding cartilage. We found that dynamic motions of gait and squatting maintained consistent fluid load support between cycles, while static single-leg stance experienced slight fluid depressurization with significant reduction of solid phase stress and strain. Presence of the labrum did not significantly influence fluid load support within the articular cartilage, but prevented deformation at the cartilage edge, leading to lower stress and strain conditions in the cartilage. A morphologically accurate representation of collagen fibril orientation through the thickness of the articular cartilage was not necessary to predict fluid load support. However, comparison with simplified fibril reinforcement underscored the physiological importance. The results of this study demonstrate that an elastic incompressible material approximation is reasonable for modeling a limited number of cyclic motions of gait and squatting without significant loss of accuracy, but is not appropriate for static motions or numerous repeated motions. Additionally, effects seen from removal of the labrum motivate evaluation of labral reattachment strategies in the context of labral repair.
2013
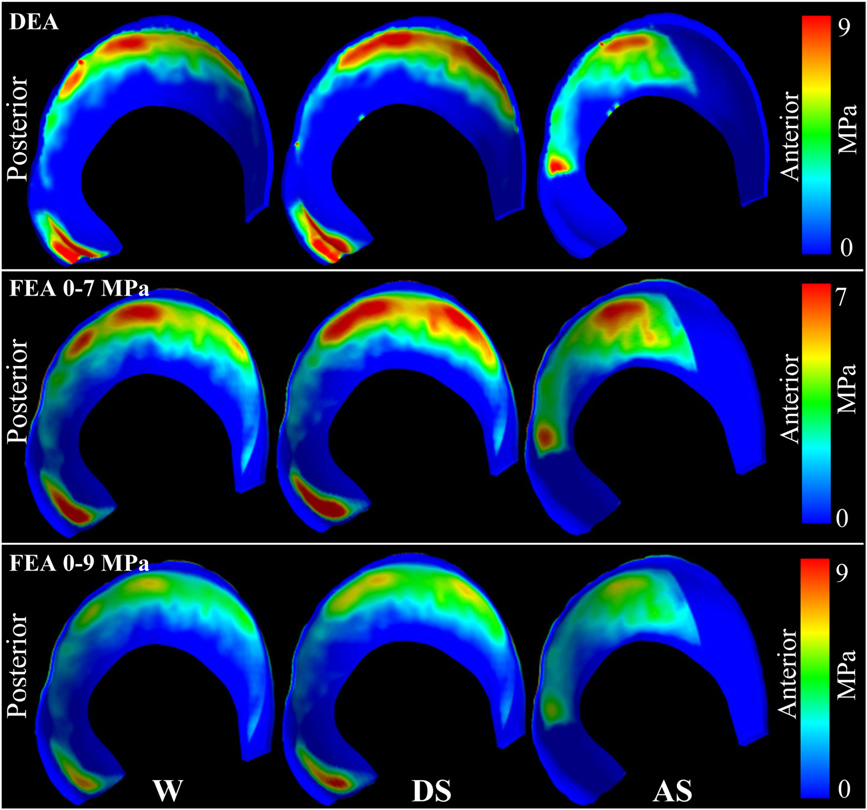
C.L. Abraham, S.A. Maas, J.A. Weiss, B.J. Ellis, C.L. Peters, A.E. Anderson.
“A new discrete element analysis method for predicting hip joint contact stresses,” In Journal of Biomechanics, Vol. 46, No. 6, pp. 1121--1127. 2013.
DOI: 10.1016/j.jbiomech.2013.01.012
C.R. Henak, A.K. Kapron, B.J. Ellis, S.A. Maas, A.E. Anderson, J.A. Weiss.
“Specimen-specific predictions of contact stress under physiological loading in the human hip: validation and sensitivity studies,” In Biomechanics and Modeling in Mechanobiology, pp. 1-14. 2013.
DOI: 10.1007/s10237-013-0504-1
Hip osteoarthritis may be initiated and advanced by abnormal cartilage contact mechanics, and finite element (FE) modeling provides an approach with the potential to allow the study of this process. Previous FE models of the human hip have been limited by single specimen validation and the use of quasi-linear or linear elastic constitutive models of articular cartilage. The effects of the latter assumptions on model predictions are unknown, partially because data for the instantaneous behavior of healthy human hip cartilage are unavailable. The aims of this study were to develop and validate a series of specimen-specific FE models, to characterize the regional instantaneous response of healthy human hip cartilage in compression, and to assess the effects of material nonlinearity, inhomogeneity and specimen-specific material coefficients on FE predictions of cartilage contact stress and contact area. Five cadaveric specimens underwent experimental loading, cartilage material characterization and specimen-specific FE modeling. Cartilage in the FE models was represented by average neo-Hookean, average Veronda Westmann and specimen- and region-specific Veronda Westmann hyperelastic constitutive models. Experimental measurements and FE predictions compared well for all three cartilage representations, which was reflected in average RMS errors in contact stress of less than 25 %. The instantaneous material behavior of healthy human hip cartilage varied spatially, with stiffer acetabular cartilage than femoral cartilage and stiffer cartilage in lateral regions than in medial regions. The Veronda Westmann constitutive model with average material coefficients accurately predicted peak contact stress, average contact stress, contact area and contact patterns. The use of subject- and region-specific material coefficients did not increase the accuracy of FE model predictions. The neo-Hookean constitutive model underpredicted peak contact stress in areas of high stress. The results of this study support the use of average cartilage material coefficients in predictions of cartilage contact stress and contact area in the normal hip. The regional characterization of cartilage material behavior provides the necessary inputs for future computational studies, to investigate other mechanical parameters that may be correlated with OA and cartilage damage in the human hip. In the future, the results of this study can be applied to subject-specific models to better understand how abnormal hip contact stress and contact area contribute to OA.
S.C. Sibole, S.A. Maas, J.P. Halloran, J.A. Weiss, A. Erdemir.
“Evaluation of a post-processing approach for multiscale analysis of biphasic mechanics of chondrocytes,” In Computer Methods in Biomechanical and Biomedical Engineering, Vol. 16, No. 10, pp. 1112--1126. 2013.
DOI: 10.1080/10255842.2013.809711
PubMed ID: 23809004
Understanding the mechanical behaviour of chondrocytes as a result of cartilage tissue mechanics has significant implications for both evaluation of mechanobiological function and to elaborate on damage mechanisms. A common procedure for prediction of chondrocyte mechanics (and of cell mechanics in general) relies on a computational post-processing approach where tissue-level deformations drive cell-level models. Potential loss of information in this numerical coupling approach may cause erroneous cellular-scale results, particularly during multiphysics analysis of cartilage. The goal of this study was to evaluate the capacity of first- and second-order data passing to predict chondrocyte mechanics by analysing cartilage deformations obtained for varying complexity of loading scenarios. A tissue-scale model with a sub-region incorporating representation of chondron size and distribution served as control. The post-processing approach first required solution of a homogeneous tissue-level model, results of which were used to drive a separate cell-level model (same characteristics as the sub-region of control model). The first-order data passing appeared to be adequate for simplified loading of the cartilage and for a subset of cell deformation metrics, for example, change in aspect ratio. The second-order data passing scheme was more accurate, particularly when asymmetric permeability of the tissue boundaries was considered. Yet, the method exhibited limitations for predictions of instantaneous metrics related to the fluid phase, for example, mass exchange rate. Nonetheless, employing higher order data exchange schemes may be necessary to understand the biphasic mechanics of cells under lifelike tissue loading states for the whole time history of the simulation.
2012
G.A. Ateshian, S.A. Maas, J.A. Weiss.
“Solute transport across a contact interface in deformable porous media,” In Journal of Biomechanics, Vol. 45, No. 6, pp. 1023-–1027. 2012.
DOI: 10.1016/j.jbiomech.2012.01.003
A finite element formulation of neutral solute transport across a contact interface between deformable porous media is implemented and validated against analytical solutions. By reducing the integral statements of external virtual work on the two contacting surfaces into a single contact integral, the algorithm automatically enforces continuity of solute molar flux across the contact interface, whereas continuity of the effective solute concentration (a measure of the solute mechano-chemical potential) is achieved using a penalty method. This novel formulation facilitates the analysis of problems in biomechanics where the transport of metabolites across contact interfaces of deformable tissues may be of interest. This contact algorithm is the first to address solute transport across deformable interfaces, and is made available in the public domain, open-source finite element code FEBio (http://www.febio.org).
Keywords: FEBio, Finite element modeling, Contact mechanics, Solute transport, Porous media, Biphasic theory
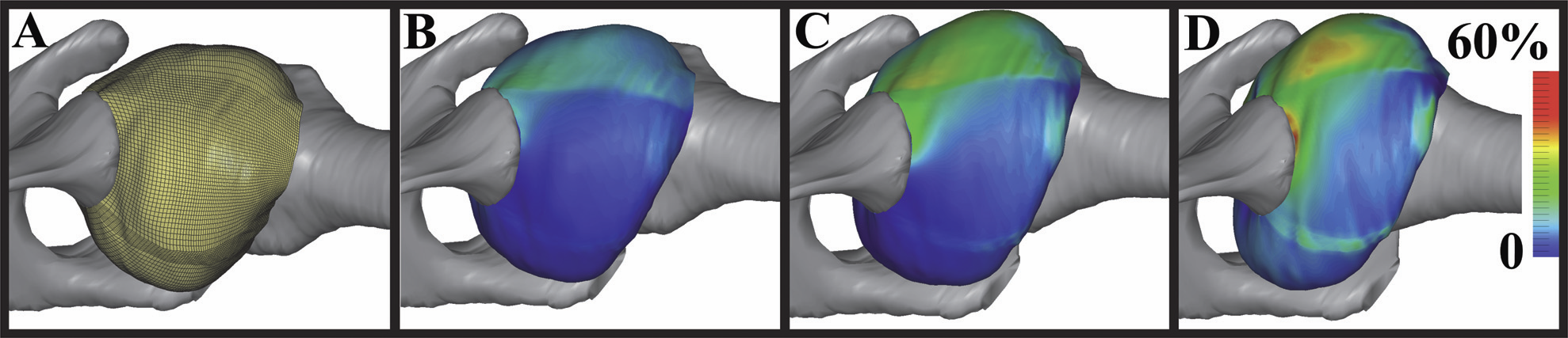
S.A. Maas, B.J. Ellis, G.A. Ateshian, J.A. Weiss.
“FEBio: Finite elements for biomechanics,” In Journal of Biomechanical Engineering, Vol. 134, No. 1, pp. 011005. 2012.
DOI: 10.1115/1.4005694
PubMed ID: 22482660
2011
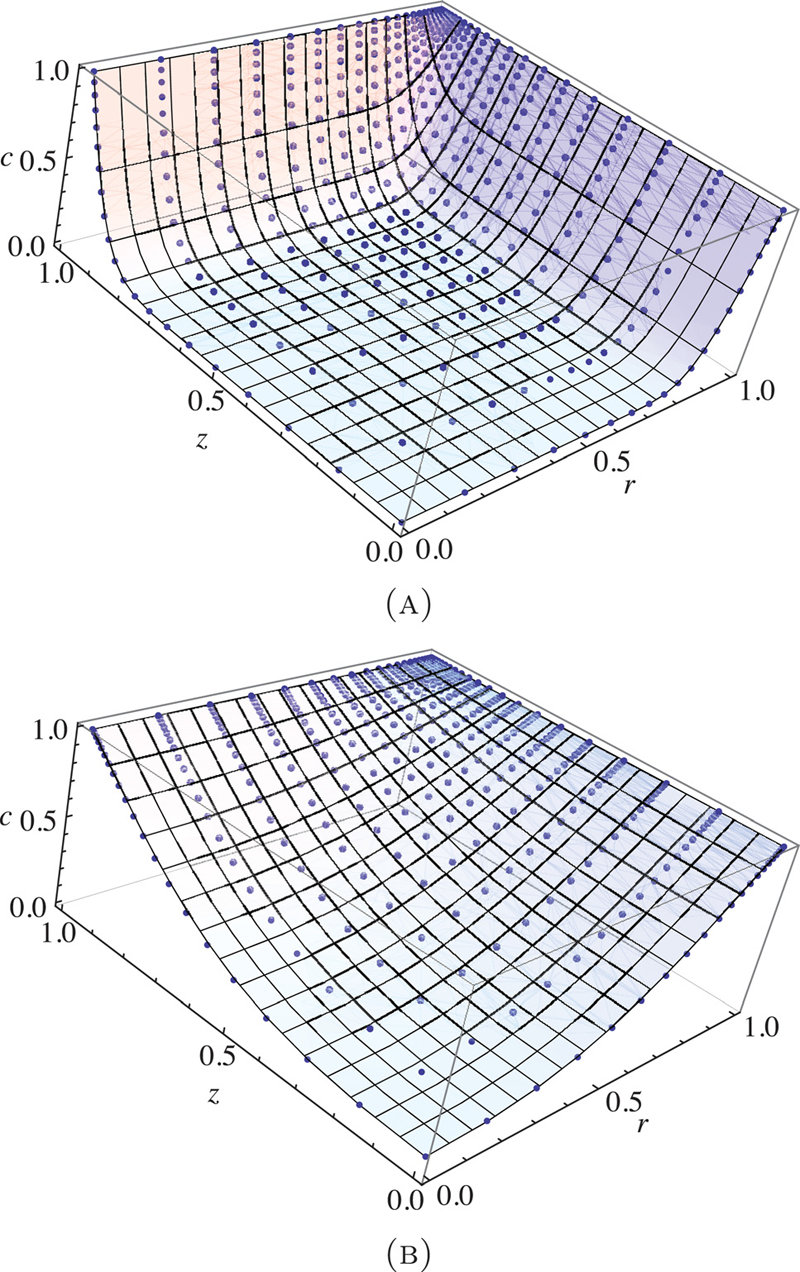
G.A. Ateshian, M.B. Albro, S.A. Maas, J.A. Weiss.
“Finite element implementation of mechanochemical phenomena in neutral deformable porous media under finite deformation,” In Journal of Biomechanical Engineering, Vol. 133, No. 8, 2011.
DOI: 10.1115/1.4004810
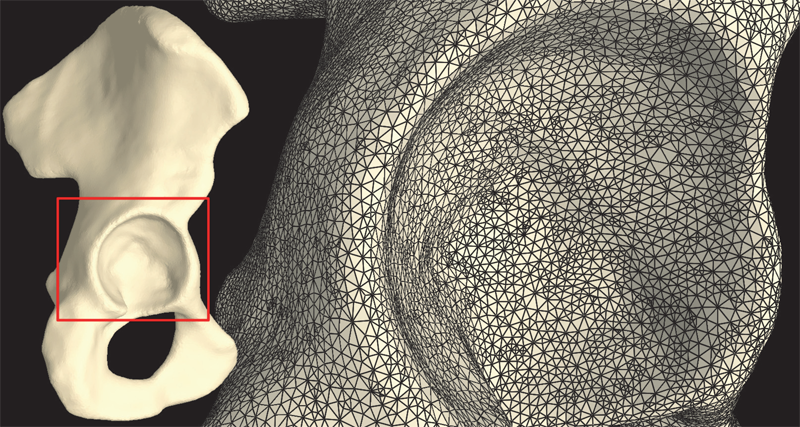
S.A. Maas, B.J. Ellis, D.S. Rawlins, L.T. Edgar, C.R. Henak, J.A. Weiss.
“Implementation and Verification of a Nodally-Integrated Tetrahedral Element in FEBio,” SCI Technical Report, No. UUSCI-2011-007, SCI Institute, University of Utah, 2011.
Keywords: MRL
2010
A.E. Anderson, B.J. Ellis, S.A. Maas, J.A. Weiss.
“Effects of idealized joint geometry on finite element predictions of cartilage contact stresses in the hip,” In Journal of Biomechanics, Vol. 43, No. 7, pp. 1351--1357. May, 2010.
Computational models may have the ability to quantify the relationship between hip morphology, cartilage mechanics and osteoarthritis. Most models have assumed the hip joint to be a perfect ball and socket joint and have neglected deformation at the bone-cartilage interface. The objective of this study was to analyze finite element (FE) models of hip cartilage mechanics with varying degrees of simplified geometry and a model with a rigid bone material assumption to elucidate the effects on predictions of cartilage stress. A previously validated subject-specific FE model of a cadaveric hip joint was used as the basis for the models. Geometry for the bone-cartilage interface was either: (1) subject-specific (i.e. irregular), (2) spherical, or (3) a rotational conchoid. Cartilage was assigned either a varying (irregular) or constant thickness (smoothed). Loading conditions simulated walking, stair-climbing and descending stairs. FE predictions of contact stress for the simplified models were compared with predictions from the subject-specific model. Both spheres and conchoids provided a good approximation of native hip joint geometry (average fitting error ∼0.5 mm). However, models with spherical/conchoid bone geometry and smoothed articulating cartilage surfaces grossly underestimated peak and average contact pressures (50% and 25% lower, respectively) and overestimated contact area when compared to the subject-specific FE model. Models incorporating subject-specific bone geometry with smoothed articulating cartilage also underestimated pressures and predicted evenly distributed patterns of contact. The model with rigid bones predicted much higher pressures than the subject-specific model with deformable bones. The results demonstrate that simplifications to the geometry of the bone-cartilage interface, cartilage surface and bone material properties can have a dramatic effect on the predicted magnitude and distribution of cartilage contact pressures in the hip joint.
Keywords: mrl
G.A. Ateshian, S.A. Maas, J.A. Weiss.
“Finite element algorithm for frictionless contact of porous permeable media under finite deformation and sliding,” In Journal of Biomechanical Engineering, Vol. 132, No. 6, Note: Cover article, 2010.
S.P. Reese, S.A. Maas, J.A. Weiss.
“Micromechanical models of helical superstructures in ligament and tendon fibers predict large poisson's ratios,” In Journal of Biomechanics, Vol. 43, No. 7, pp. 1394--1400. 2010.
2009
H.B. Henninger, S.A. Maas, J.H. Shepherd, S. Joshi, J.A. Weiss.
“Transversely Isotropic Distribution of Sulfated Glycosaminoglycans in Human Medial Collateral Ligament: A Quantitative Analysis,” In Journal of Structural Biology, Vol. 165, pp. 176-183. 2009.
PubMed ID: 19126431
S.A. Maas, B.J. Ellis, D.S. Rawlins, J.A. Weiss.
“A Comparison of FEBio, ABAQUS, and NIKE3D Results for a Suite of Verification Problems,” SCI Technical Report, No. UUSCI-2009-009, SCI Institute, University of Utah, 2009.
N.S. Phatak, S.A. Maas, A.I. Veress, N.A. Pack, E.V. DiBella, J.A. Weiss.
“Strain Measurement in the Left Ventricle During Systole with Deformable Image Registration,” In Medical Image Analysi, Vol. 13, No. 2, pp. 354--361. 2009.
E.J. Rainis, S.A. Maas, H.B. Henninger, P.J. McMahon, J.A. Weiss, R.E. Debski.
“Material properties of the axillary pouch of the glenohumeral capsule: Is isotropic material symmetry appropriate?,” In Journal of Biomechanical Engineering, Vol. 131, No. 13, pp. (7 pages). 2009.
2008
A.E. Anderson, B.J. Ellis, S.A. Maas, C.L. Peters, J.A. Weiss.
“Validation of Finite Element Predictions of Cartilage Contact Pressure in the Human Hip Joint,” In ASME Journal of Biomechanical Engineering, Vol. 130, No. 3, pp. 051008-1--10. May, 2008.
L. Krishnan, C.J. Underwood, S.A. Maas, B.J. Ellis, T.C. Kode, J.B. Hoying, J.A. Weiss.
“Effect of Mechanical Boundary Conditions on Orientation of Angiogenic Microvessels,” In Cardiovascular Research, Vol. 78, No. 2, pp. 324--332. 2008.
N.S. Phatak, S.A. Maas, A.I. Veress, N.A. Pack, E.V.R. Di Bella, J.A. Weiss.
“Strain measurement in the left ventricle during systole with deformable image registration,” In Medical Image Analysis, pp. (in press). 2008.
Page 1 of 2
