Therefore, the objective of this collaboration is to build upon the previous, well proven, clinical success of electrically induced bone growth used to augment fracture healing and expand this technology to accelerate osseointegration in the percutaneous model for amputees. Since osseointegration technology is still fairly new for lower extremity amputees and not utilized clinically in the United States, ways to increase its efficiency are still developing. The CIBC collaborator is addressing this limitation by developing an Osseointegrated Intelligent Implant Design (OIID) system which has been awarded a United States provisional patent with the University of Utah Technology Commercialization Office as a novel rehabilitation tool to improve osseointegration technology. The addition of electrical stimulation may increase the rate, magnitude, and quality of initial skeletal attachment to the osseointegrated prosthetic stem.
To validate the general hypothesis that electrical stimulation will increase skeletal attachment, a two phase project has been designed that utilizes in vitro, in vivo, and in silico modalities to confirm the safety and efficacy of this technology prior to implementation in amputees. The specific hypotheses for this model are founded on histological assessment, mechanical testing, and finite element analysis. The research hypothesis from the Bloebaum group that is most relevant to the Center is that, "Finite element based simulation analysis of veteran and warrior amputee residual limbs imaged with computed tomography scans will reveal that safe and effective current densities and electric fields will be attainable at the bone-implant interface."
To evaluate this hypothesis by creating the necessary simulation infrastructure, the Center has been working toward the following technical aims:
Aim 1: Develop segmentation and mesh generation support that is adapted to orthopedic applications like this one and to facilitate the rapid generation of accurate geometric models of amputees and the implants and stimulation electrodes required for these modeling applications.
Aim 2: Accelerate the computations required to simulate electric fields and current densities for any selected boundary conditions of implant and skin surface electrodes and provide extensive visualization of the results that include certainty and parameter sensitivity.
Aim 3: Develop estimation and optimization strategies for locating skin surface electrodes in ways that maximize the growth potential for electrical stimulation of osseointegration.
Recent Progress
Within this field, poor prosthetic fit is often the result of heterotopic ossification (HO), a frequent problem following blast injuries for returning service members. Osseointegration technology offers an advantage for individuals with significant HO and poor socket tolerance by using direct skeletal attachment of a prosthesis to the distal residual limb, but remains limited due to prolonged post-operative rehabilitation regimens. Therefore, electrical stimulation has been proposed as a catalyst for expediting skeletal attachment and the bioelectric effects of HO were evaluated using finite element analysis in 11 servicemen with transfemoral amputations. Retrospective computed tomography (CT) scans provided accurate reconstructions, and volume conductor models demonstrated the variability in residual limb anatomy and necessity for patient-specific modeling to characterize electrical field variance if patients were to undergo a theoretical osseointegration of a prosthesis. In this investigation, the volume of HO was statistically significant when selecting the optimal potential difference for enhanced skeletal fixation, since higher HO volumes required increased voltages at the periprosthetic bone (p = 0.024, r = 0.670). Results from Spearman's rho correlations also indicated that the age of the subject and volume of HO were statistically significant and inversely proportional, in which younger service members had a higher frequency of HO (p = 0.041, r = −0.622). This study demonstrates that the volume of HO and age may affect the voltage threshold necessary to improve current osseointegration procedures.The CT files collected during the study were imported into Seg3D. The tissue boundaries of the internal organs, bone, bone marrow, and adipose tissue were generated by thresholding the CT files interactively using fixed thresholded values for all of the patients' CTs. Musculature was obtained by manually setting seed points inside the tissue and using a confidence connected filter to find all the tissue connected to the seed points since the complex geometry required additional image processing. Because the skin was impossible to discern reliably from CT images, an estimate of the skin layer was generated by dilating the outermost visible tissue to produce a 2-mm layer of homogeneous thickness (the average thickness of human skin) to surround the full model.
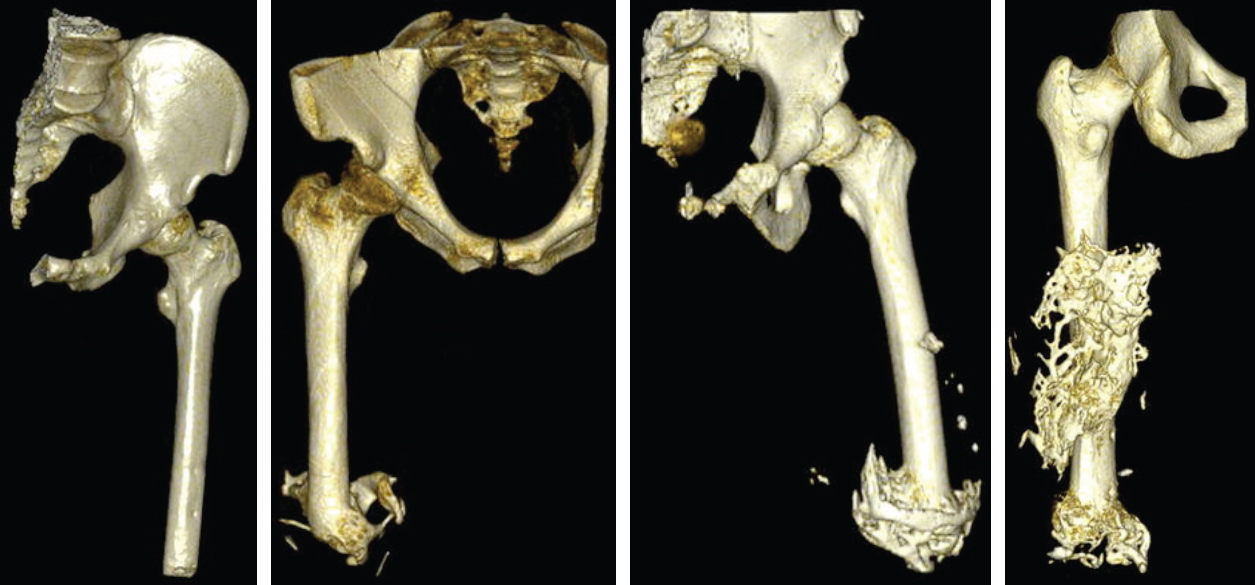 |
| Figures from B.M. Issacson et al., Three dimensional reconstruction of a service members with (from left to right) with no visible signs of HO; 26.53 cm3 of HO; 74.25 cm3 of HO; and 115.96 cm3 of HO |

