 |
| Overview of the DBS system. The DBS electrode is implanted in the brain during stereotactic surgery. The electrode is attached via an extension wire to the IPG, which is implanted in the torso. The entire system is subcutaneous and is designed to deliver continuous stimulation for several years at a time. |
The selection of DBS settings is a significant clinical challenge that requires repeated revisions to achieve optimal therapeutic response, and is often performed without any visual representation of the stimulation system in the patient. We used ImageVis3D Mobile to provide models to movement disorders clinicians and asked them to use the software to determine: 1) which of the four DBS electrode contacts they would select for therapy and 2) what stimulation settings they would choose. We compared the stimulation protocol chosen from the software versus the stimulation protocol that was chosen via clinical practice (independent of the study). Lastly, we compared the amount of time required to reach these settings using the software versus the time required through standard practice. We found that the stimulation settings chosen using ImageVis3D Mobile were similar to those used in standard care, but were selected in drastically less time. We found that our visualization system, available directly at the point of care on a device familiar to the clinician, can be used to guide clinical decision-making for selecting DBS settings. The positive impact of the system could also translate to areas other than DBS.
In order to evaluate the utility of ImageVis3D Mobile for clinical decision making, we constructed patient-specific models of four PD patients who were good responders to DBS. Models were created in SCIRun2 and subsequently transferred to ImageVis3D Mobile. We provided these models to five clinicians (three movement disorders neurologists, one neurosurgeon, and one nurse) who have extensive experience with programming DBS systems for PD patients. Each clinician was asked to select DBS parameters using ImageVis3D Mobile on an iPad without knowing the identity of the patients. We then compared their selections to data collected via standard of care, along with the amount of time required. In our study we compared the amount of time required to perform initial DBS programming to the amount of time required to choose stimulation parameters using IV3D. The time required via standard care was estimated from retrospective chart review. In addition, we compared the stimulation settings chosen using each approach.
We found that the amount of time required to choose stimulation settings was significantly less using ImageVis3D Mobile compared to standard clinical care. The selection of stimulation settings required an average of 1.7 +/- 0.8 minutes per patient across all clinicians, compared to an average of 4 +/- 1.4 hours required for programming via standard of care to reach stable settings with good therapeutic response (usually within three to four clinic visits). Moreover, we found that the stimulation settings chosen using ImageVis3D Mobile were very similar to those selected via standard of care. The voltages selected using ImageVis3D Mobile were generally equal to or smaller than the voltages selected using standard care, and in fact this is a trend that had been observed previously3.
The active electrode contacts chosen using ImageVis3D Mobile were either the same as or adjacent to the contact chosen using standard care. Prior studies have noted comparable therapeutic benefits from more than one electrode contact4. Hence, we consider this degree of variability to be within the range that is observed clinically.
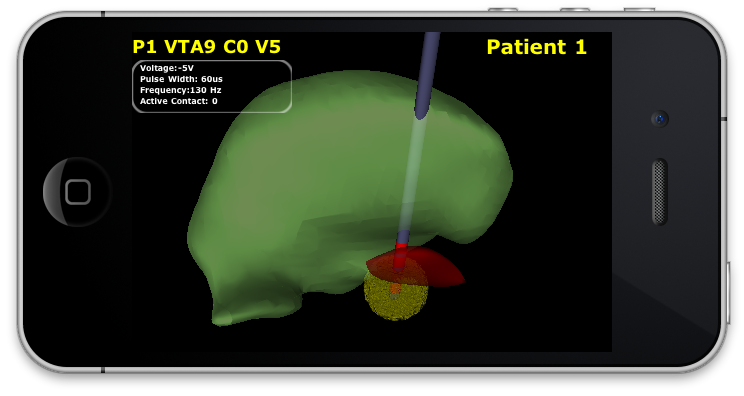 |
| Interleaved view in ImageVis3D Mobile. The VTA for -2.5V at contact 3 is shown for a specific patient. |
We anticipate that this system could provide a significant step forward in clinical practice for several reasons: mobile computing platforms such as the iPhone are widely used by physicians, and new hardware devices such as the iPad have generated significant interest in the clinical community; computational models are gaining acceptance by practitioners and are being used more often for clinical decision-making; the system described here has a simple, intuitive interface that can be used at the patient's bedside. A final advantage of this approach is more subtle. In the course of the experiment, we realized that the interactive visualization provided a structure for comparing different approaches to DBS programming. One persistent problem in neuromodulation is that the vocabulary for describing target locations is somewhat imprecise, and alternate programming approaches are employed by different practitioners.
While previous attempts have been made to provide interactive visualization of patient-specific DBS models, these require significant amounts of training and domain knowledge to become proficient. An advantage of the system described here is the minimal amount of training required and its attractive features for clinical workflow. We predict that this approach could have a significant impact not only in DBS for PD, but also in other neuromodulation methods where interactive patient specific models could provide useful insights into the best way to prescribe the therapy. We conclude that the use of patient specific models of DBS in a mobile computing device running ImageVis3D Mobile has strong potential to improve patient outcomes by facilitating clinical decision-making.
References
[1] C.R. Butson, G. Tamm, S. Jain, T. Fogal, J. Kruger, Evaluation of Interactive Visualization on Mobile Computing Platforms for Selection of Deep Brain Stimulation Parameters, IEEE Transactions on Visualization and Computer Graphics.[2] Scientific Computing and Imaging (SCI) Institute. SCIRun: A scientific computing problem solving environment. http://www.scirun.org.
[3] A. M. Frankemolle, J. Wu, A. M. Noecker, C. Voelcker-Rehage, J. C. Ho, J. L. Vitek, C. C. McIntyre, and J. L. Alberts. Reversing cognitivemotor impairments in parkinson's disease patients using a computational modelling approach to deep brain stimulation programming. Brain, 133(Pt 3):746‚61, 2010. http://brain.oxfordjournals.org/cgi/content/abstract/ awp315v1.
[4] M. S. Okun, H. H. Fernandez, S. S. Wu, Kirsch-Darrow, B. L., B. D., F., M. Suelter, C. E. Jacobson, X. Wang, C. W. Gordon, P. Zeilman, J. Romrell, P. Martin, H. Ward, R. L. Rodriguez, and K. D. Foote. Cognition and mood in parkinson's disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Annals of Neurology, 65:586‚95, 2009.
