 Growth of new blood vessels from existing ones. |
| Day 0 To observe angiogenesis, a blood vessel fragment has been isolated and placed in a collagen gel. |
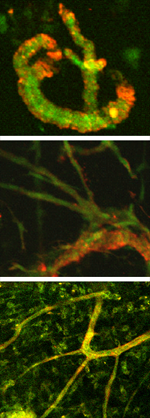 |
| Day 8 The vessel fragment has sprouted several capillary branches. |
|
| Day 28 The vessel construct has been implanted into live tissue and is supplying blood. |
An aspect currently under investigation deals with how the infiltration of blood vessels affects the material properties of the tissues. The working hypothesis is that tissues are weakened as the density of blood vessels increases. MRL researchers are testing this hypothesis by growing networks of blood vessels in a collagen gel substrate. At fixed time points during angiogenesis, the material properties of the vascularized collagen scaffold are characterized to test its rigidity and subject the growing blood vessels to stress.
The relationships between globally applied stresses and strains and the resulting stresses and strains experienced at the tips of the growing capillaries are being investigated using a computational mechanics methodology called the Material Point Methods (MPM). The software framework was largely developed under another SCI Institute collaboration project, The Center for the Simulation of Accidental Fires and Explosions (C-SAFE). This method simulates any material with a series of points that have a number of properties. In this case, MPM works by modeling the collagen lattice and growing capillaries with a number of points on a background grid. Each point carries information about the mechanical properties of the tissue it represents including it's motion and how strongly it is bound to the neighboring tissues. As the simulation progresses, the grid is stretched to represent the mechanical stress being imposed on the tissues and the points are carried along with it. The grid is reset to an undeformed state on each cycle but the points are left at their new locations and the process is repeated. Material Point Methods have proven to be an efficient and effective way to simulate all kinds of mechanical systems.
 |
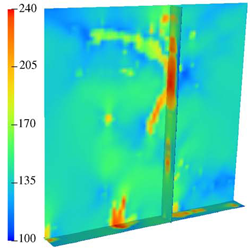 |
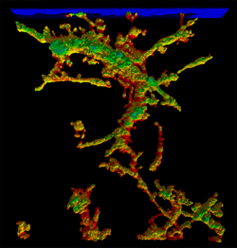
| An MPM simulation of a microvessel undergoing axial extension. Red indicates areas of highest stress load. (collagen not shown for clarity) | Distribution of von Mises stress (Pa) for the 3D model under 10% axial extension. Note the highly inhomogeneous stress distribution and the vertical channeling of stresses through the microvessels. |
This multidisciplinary collaborative project is currently funded by the National Institutes of Health (NHLBI R01 HL077683).
Principal Researchers
- Jeff Weiss - PI, University of Utah
- Jim Guilkey - University of Utah
- Jay Hoying - University of Arizona
- Urs Utzinger - University of Arizona
B.R. Shepherd, H.Y. Chen, C.M. Smith, G. Gruionu, S.K. Williams, and J.B. Hoying. "Rapid Perfusion and Network Remodeling in a Microvascular Construct after Implantation," In Arterioscler Thromb Vasc Biol. Vol. 24, No. 5, pp. 898--904, 2004.Versions Available: [ PubMed ]
J.B. Hoying and C.A. Boswell and S.K. Williams. "Angiogenic Potential of Microvessel Fragments Established in Three-Dimensional Collagen Gels,"In Vitro Cell Dev Biol Anim Vol. 32, No. 7, pp. 409--419, 1996.Versions Available: [ PubMed ]
