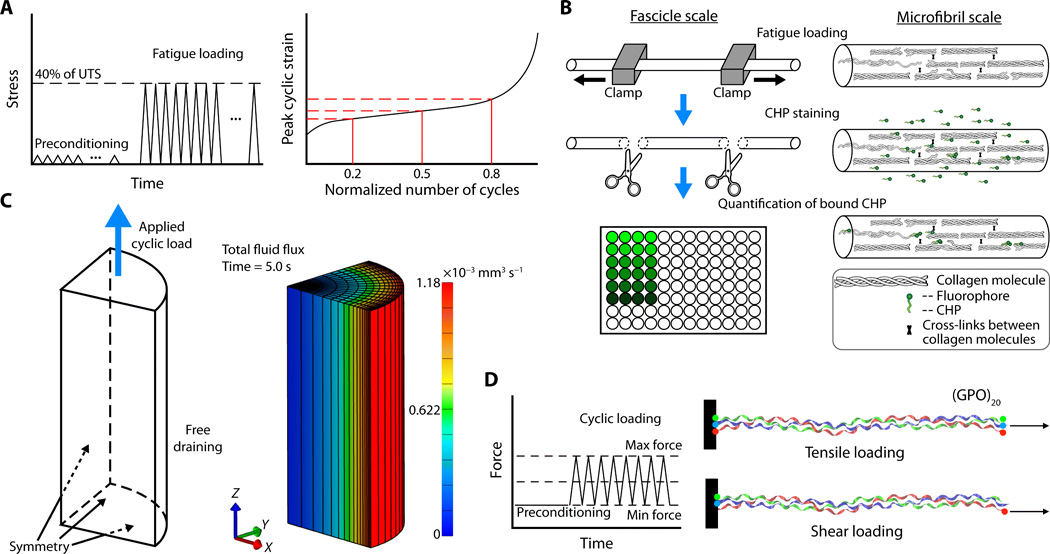
Accumulation of collagen molecular unfolding is the mechanism of cyclic fatigue damage and failure in collagenous tissues
In understanding the failure of dense collagenous soft tissues over multiple loading cycles, the predominant hypothesis for development of overuse injuries is that repeated subfailure loading causes accumulation of “micro-damage”, and when this micro-damage accumulates at a rate that is faster than can be repaired, this results in injury in a clinical sense (tissue failure and resulting pain from the injury and overload of surrounding structures). However the specific nature of this micro-damage has remained unknown. In this study, we demonstrate that the micro-damage is actually collagen molecular unfolding, which accumulates with repeated cyclic loading. Our results provide a convincing explanation for the micro-damage hypothesis: Molecular-level collagen damage is generated by tissue-level loading, and the ability to repair this damage determines whether the applied loading leads to tissue failure.You can read the full paper in Science Advances